
 Above: Jennifer Landa
Above: Jennifer Landa
Written By: Sara Daniels
Photos By: John Packman
At age 26, Jennifer Landa was the picture of radiant health. A longtime runner, she led a healthy and active lifestyle and was full of boundless energy. She had passed her recent physical with flying colours.
Then, a few months later, Jennifer discovered a lump in her breast during a regular self-examination. She wasn’t worried, but she knew she should have it checked out because breast cancer was common in her family — both her maternal and paternal grandmothers and one of her grandfathers were diagnosed with and treated for the disease. And because of their family history, her mother had been monitored for years by the team at the Marvelle Koffler Breast Centre (MKBC) at Mount Sinai Hospital, part of Sinai Health System.
After an initial consultation and ultrasound with her doctor, Jennifer’s mother encouraged her to come to MKBC, Canada’s first dedicated multidisciplinary breast centre, for a second ultrasound. Jennifer took her mother’s advice and made an appointment, expecting a routine procedure.
Instead, the technician discovered three lumps: two in her breast, and a swollen lymph node. A surgeon consulted on the ultrasound and ordered a mammogram, MRI and biopsy right away. Amid the flurry of activity and concern, Jennifer realized something was clearly wrong. She was diagnosed with stage 3 breast cancer, and learned that she had inherited a genetic mutation, known as BRCA2, that predisposes individuals who carry it to developing breast and ovarian cancers.
“I was in disbelief,” Jennifer recalls. “It came out of nowhere.”
Dr. Pamela Goodwin, Jennifer’s physician and Director of the MKBC, quickly implemented a treatment plan. Within days of her diagnosis, Jennifer began her first round of chemotherapy. Her treatment continued for the next year and a half, involving surgery and multiple rounds of chemotherapy and radiation. In 2015, Jennifer was given the all-clear by her doctors.
Feeling grateful and energized, Jennifer jumped back into her very full life, spending time with her huge network of friends and family who had been such an incredible support system during her treatment. And she eagerly sought out ways to share her story and support Sinai Health Foundation in its mission to raise money for the MKBC to help other patients facing breast cancer diagnoses.
After more than a year of living cancer-free, Jennifer came back to Mount Sinai in August 2016 because she was having trouble breathing. A CT scan revealed that her cancer had returned, and had spread to her sternum, right rib cage, spine and right pelvis. Her doctors developed a treatment plan designed to slow the spread of her cancer and give her as much time as possible. But her cancer was clearly aggressive, and they warned her that she might not live more than another five years.
As Jennifer came to terms with this new diagnosis, she remained positive, continuing to live every day to the fullest and even making a bucket list of goals she wanted to achieve. Then, in early October, while at a Maple Leafs game with a friend, she began to experience unusual tremors in her left arm and leg. Within an hour she was vomiting and could barely walk. Her elder brother Michael brought her to the emergency room at Mount Sinai, where clinical staff gave her anti-nausea medication to ease her vomiting. Rather than pursue further tests that night, she decided to wait for her previously scheduled appointment with Dr. Goodwin on the upcoming Tuesday.
 Above: Jennifer speaking at Oakdale Golf fore the Cure in July 2015. Photo credit: Rosemary Goldhar
Above: Jennifer speaking at Oakdale Golf fore the Cure in July 2015. Photo credit: Rosemary Goldhar
After working from home on Monday, Jennifer came in for her appointment with her arm and leg still twitching uncontrollably. Dr. Goodwin immediately ordered a CT scan and admitted her to the hospital, where she also had an urgent MRI. The twitching, the tests revealed, was due to a localized seizure caused by one of 10 tumours in her brain, the largest 3.5 cm in size.
After a few days in the hospital, Jennifer returned home, grappling with her latest diagnosis. Six weeks before, she thought she might live only another five years; now she learned she had just months left.
Breast cancer researcher Dr. Daniel Schramek, Kierans/Janigan Cancer Research Scientist at the Lunenfeld-Tanenbaum Research Institute (LTRI), is looking at ways to treat — and even prevent — hormone-induced breast cancers like Jennifer’s. During his doctoral work with Dr. Josef Penninger at the Austrian Academy of Sciences in his native Vienna, he discovered that rank ligand (RANKL), a molecule that affects the immune system and controls bone development, plays a key role in driving hormone-induced breast cancer.
In the mammary glands, RANKL causes cells to grow and divide rapidly, for example to induce lactation after a woman gives birth so she can breastfeed. Dr. Penninger suspected the function of RANKL in the mammary gland may have evolved to help pull calcium from women’s bones to provide this nutrient to babies in breast milk. RANKL’s dual function in balancing calcium during bone development and lactation (i.e., bone destruction) might also be the reason why women are also much more likely to develop osteoporosis, a condition resulting in brittle bones.
If breast tissue cells have the ability to destruct bone — to release calcium into breast milk — so too might breast cancer cells. But instead of releasing calcium, the cancer cell degrades the bone and divides again and again, causing metastasis of the original breast cancer tumour to the bone. This could help explain why breast cancer often spreads to the bones, particularly the rib cage and spine, as Jennifer’s cancer has done.
Dr. Schramek’s early research, published in Nature in 2010, demonstrated that blocking the RANKL gene in patients with hormone-induced breast cancer could be an effective new approach to treatment. Now, clinical studies that build off of his important discoveries are showing that a RANKL-blocking drug called denosumab — originally approved by the U.S. Federal Drug Administration (FDA) to treat osteoporosis — seems to also work in breast cancers.
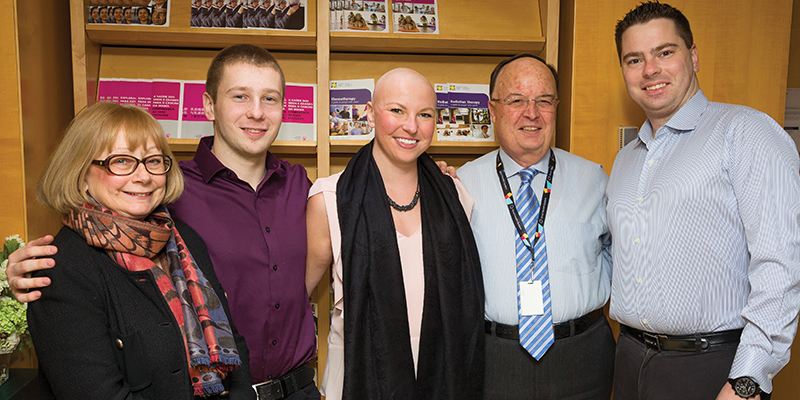 Above: Jennifer's fundraising campaign launch event in December 2016: From left, Dr. Pamela Goodwin, Christopher Landa, Jennifer, Dr. Jaime Escallon and Michael Landa. Photo credit: Doug Nicholson
Above: Jennifer's fundraising campaign launch event in December 2016: From left, Dr. Pamela Goodwin, Christopher Landa, Jennifer, Dr. Jaime Escallon and Michael Landa. Photo credit: Doug Nicholson
Dr. Schramek also hopes that using denosumab to block RANKL preventatively in patients who have a high risk of developing breast cancer could actually protect them from getting cancer at all. And as a bonus, the drug would also protect patients from osteoporosis. Testing this theory in a mouse model that was bred with an inherited BRCA gene mutation, and thus an increased risk of developing breast cancer, Dr. Schramek worked with Dr. Penninger and his team to use an antibody to block RANKL — and found that it did indeed protect the mouse from getting breast cancer. The results from this study were just published in 2016, and Dr. Schramek, a co-author on the study, is excited by the potential of this approach: Because it’s already cleared the FDA’s safety hurdles, denosumab could be used in the clinic to treat breast cancer patients within just a few years.
“Usually if you find a promising treatment in the lab, it takes 15 years before it’s available for clinical use,” Dr. Schramek explains. “If denosumab works, it could be available to patients in just a few years. That’s really, really exciting.”
Since coming to the LTRI in 2015, Dr. Schramek has joined the Hold‘em for Life Poker Charity Challenge project, a major breast cancer research initiative supported by the real estate, asset management and mining industries of Canada. Established by Dr. Goodwin, the team comprises scientists including Dr. Jim Woodgett, Koffler Director of the LTRI, and Dr. Rama Khokha, an oncologist and Senior Scientist at the Princess Margaret Cancer Centre whose work has explained the exact role of RANKL in mammary stem cell biology. The Hold‘em for Life team has recently launched a long-term study driven by clinical observations in the MKBC that most breast cancer deaths now seem to come from late recurrence — as many as 10 years after entering remission — in patients whose earlier cancer was treated effectively.
Nobody yet knows what drives late recurrence in these patients, but doctors at MKBC have observed that sometimes they have had some sort of injury — for example, a car accident, a broken bone, a surgery. At the site of the injury, the breast cancer relapses. The new Hold‘em for Life study, which will follow current breast cancer patients over the next ten years, will explore whether the data supports this theory.
Meanwhile, Dr. Schramek is taking the problem back to his mouse models. He suspects RANKL is again a likely culprit in driving late recurrences in patients who have experienced some kind of injury or insult to the body.
“If a bone breaks, it has to be remodeled to heal. That remodeling always involves RANKL, and if you have a dormant cancer cell hanging around in the body, and it is exposed to a signal that tells usually dormant cells, for example, bone cells, to grow to heal a wound, that same signal could also awaken the sleeping cancer cell. An inflammatory wounding environment is a very rich environment for cancer to grow.”
This seamless integration of clinical and basic research demonstrates the value of having the LTRI’s scientists so closely aligned with their clinical colleagues at Mount Sinai.
“I need to talk to the clinicians,” says Dr. Schramek. “I would have never started this late recurrence project if I were not educated by fellow scientists and clinicians such as Dr. Goodwin and Dr. Khoka about the problems that affect their patients.”
The early onset of Jennifer’s illness is unusual. While one in 9 Canadian women will develop breast cancer during her lifetime, typically the disease affects women in their late 50s and early 60s. Younger patients like Jennifer account for a much smaller percentage of hormone-induced breast cancers.
And yet younger women are more prone to developing a small but deadly subtype of the disease: triple negative breast cancer. Accounting for only 10 to 20 per cent of women who get breast cancer, triple negative breast cancer occurs at higher rates in women of colour and those who have the BRCA1 and BRCA2 mutations. Triple negative breast cancer patients are typically under 40, and the disease often appears in young mothers, shortly after breastfeeding.
Moved by the relative youth of the patients affected by this aggressive disease — and the lack of targeted treatment options for these women — Dr. Schramek is devoting much of his current work to learning more about how this devastating disease develops and what treatment approaches might be effective.
“The triple negative group is a small one,” says Dr. Schramek, “but it’s a heartbreaking group.”
Triple negative breast cancer is so called because these cancer cells lack receptors for the three most common drivers of breast cancer: estrogen, progesterone and human epidermal growth fact receptor 2 (HER2). Drugs developed to block these receptors to prevent cancer growth don’t work for these patients.
Dr. Schramek’s research, funded in part by Susan G. Komen, a leading breast cancer organization in the U.S., is focused on figuring out what drives the growth of these tumours. While genome-sequencing techniques have made it possible for scientists to identify mutations associated with a disease, it can’t pinpoint which of these mutations actually cause the disease. To determine that, geneticists must take these sequencing insights and test them in model organisms whose cells behave similarly to human cells.

For Dr. Schramek, the tool of choice is the mouse model. “I’m absolutely convinced that we need genetic mouse models to understand how breast cancer behaves in the body,” he says. “Cancer cell lines, which are restricted to a plastic plate and grown in an incubator, can’t tell you, for example, how a tumour spreads.”
Sequencing of triple negative breast cancer cells have not uncovered mutations that regularly appear from tumour to tumour. What it has shown is an increase in copy number alterations, suggesting that the tumours are genomically instable, and that some parts of the cells’ chromosomes and genes are repeated, while others are missing.
Dr. Schramek and his team are using mouse models to explore the role these alterations may have on triple negative tumour development. His team, including graduate student Ellen Langille, recreates the same deletions and amplifications pinpointed through sequencing in the mouse using a revolutionary gene-editing technology called clustered regularly interspaced short palindromic repeats (CRISPR). CRISPR allows scientists to make targeted genetic alterations in actual tissue, and to evaluate the effects of many distinct alterations simultaneously.
The CRISPR technology, developed by scientists just five years ago, is an extraordinary advance for the field of genetics. In the past, geneticists could make a single genetic alteration in the germline of each mouse and then breed the mice to see the alteration expressed in the next generation. It took about two years and cost $100,000 to explore just one alteration, which may or may not prove to be a cause of disease. With the development of CRISPR, which allows scientists to literally cut the DNA at a chosen point to remove and/or insert targeted DNA, Dr. Schramek’s team has developed a unique methodology allowing them to make up to 600 genetic alterations just in the mammary glands of the mouse within five weeks, at one-tenth of the cost.
This means Dr. Schramek and his team can cover a lot of genetic ground in a much shorter period of time, and they can more quickly pursue further studies of molecules and mechanisms that prove likely to play a role in causing the disease. The sooner some of the mechanisms of the disease can be identified, the sooner Dr. Schramek’s team can begin searching for targeted treatments that will help stop the disease in its tracks.
And time is of the essence for women who are facing a triple negative breast cancer diagnosis. While chemotherapy, radiation and surgery can be successful in treating these tumours, the recurrence rate for the disease is especially high. Many patients relapse in as few as two or three years, and when they do, the cancer often spreads quickly from the breast to the lungs or the brain.
Despite Jennifer’s devastating diagnosis, she has been characteristically positive and eager to accomplish as much as possible in the coming months.
“It’s all about the mini-victories that I can accomplish in the time I have left,” she says.
Chief among her goals is to share her story and the experience she has had as a patient at Mount Sinai to help raise money for breast cancer treatment and research. She is deeply grateful for everyone on her care team at MKBC who has been with her, taking care of her and fighting alongside her, every step of the way.
 “It’s the worst-case scenario, best case experience,” says Jennifer. “Everyone at Mount Sinai has been amazing. I don’t have to think about what appointment to book next, they just organize everything. I walk in and everyone knows me by first name, they’re pulling my wristband out of the drawer as soon as they see me. They don’t just treat me like that, they treat everyone like that.”
“It’s the worst-case scenario, best case experience,” says Jennifer. “Everyone at Mount Sinai has been amazing. I don’t have to think about what appointment to book next, they just organize everything. I walk in and everyone knows me by first name, they’re pulling my wristband out of the drawer as soon as they see me. They don’t just treat me like that, they treat everyone like that.”
“Jennifer has been a shining light,” says Dr. Goodwin. “She is strong and gracious and she has always chosen to focus on the positive, even when she is not feeling well. She is much loved and appreciated by the staff and other patients for her sense of humour, her joie de vivre and her unfailing ability to positively impact everyone around her. She has become an important spokeswoman and champion for our breast centre, the LTRI and for countless other patients living with this unpredictable disease.”
In November, Jennifer and her brothers starred in a powerful video for Dinner with Scientists, presented by PearTree Financial, Sinai Health Foundation’s marquee fundraising event in support of the LTRI. She also attended the event — wearing a glamourous gown chosen for the special occasion — with her brothers, where they saw the debut of the video alongside the event’s 400 guests.
Jennifer is also leading her own fundraising campaign to raise money through her vast network of loved ones and supporters. To date, she has raised more than 75 per cent of her $150,000 goal to support an endowed visiting professorship that will allow the MKBC to bring international breast cancer leaders to Mount Sinai on a regular basis to ensure that the Centre remains at the forefront of cancer treatment and research.
Ultimately, Jennifer hopes her fundraising campaign will help bring hope to future patients.
“I want to help save other people,” says Jennifer. “That would be so cool. I think that’s the most amazing thing I can do with the time I have left.”